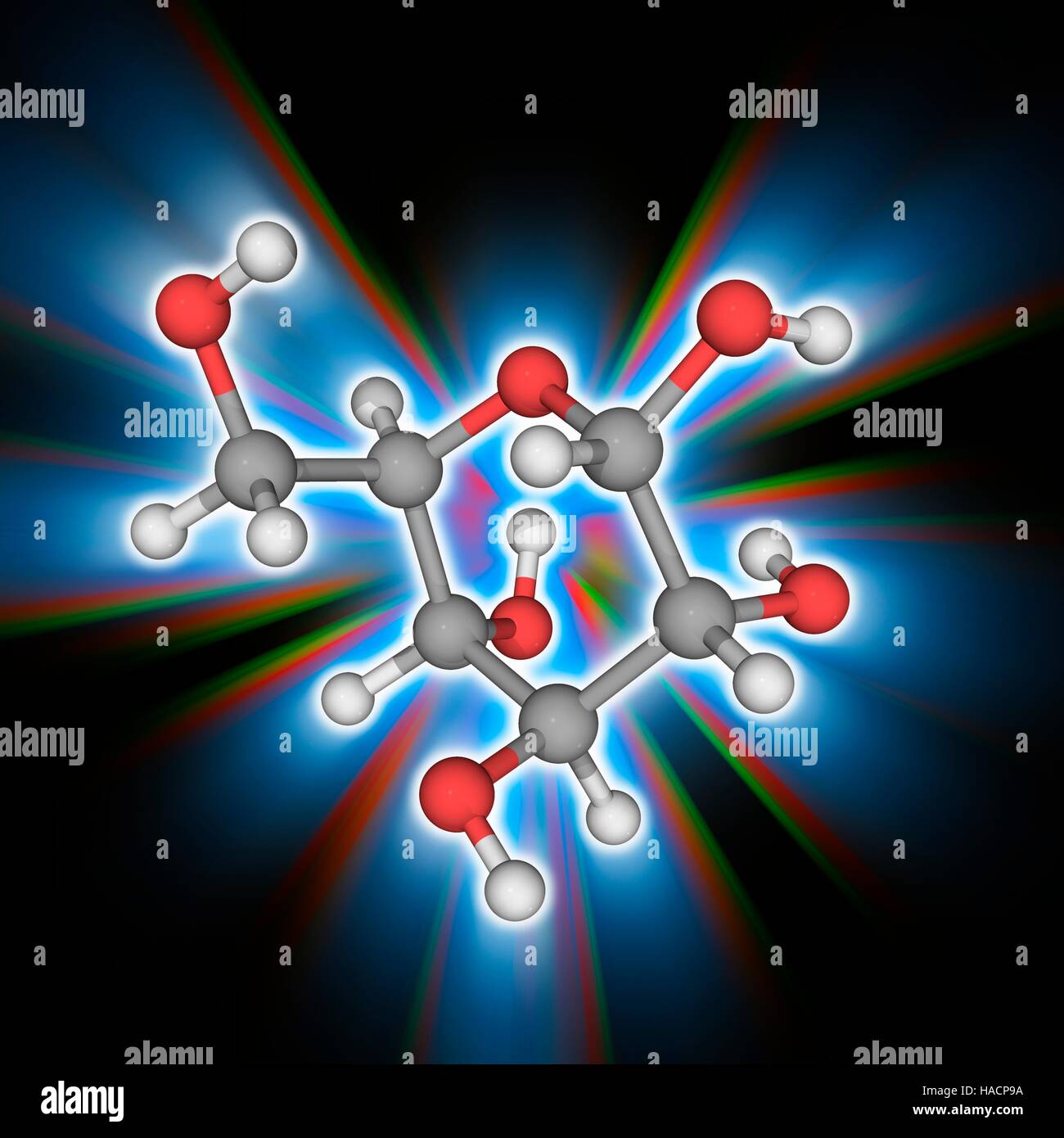
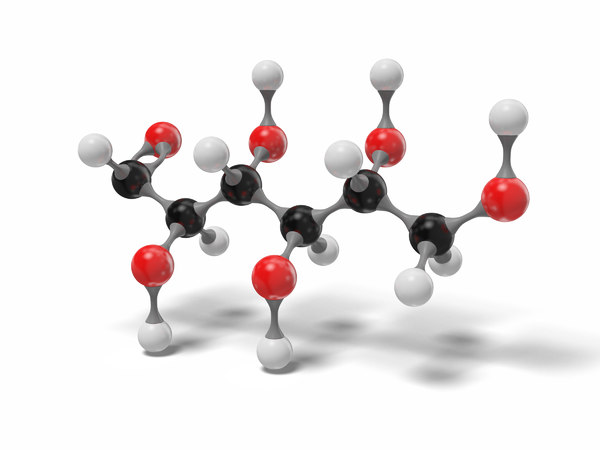
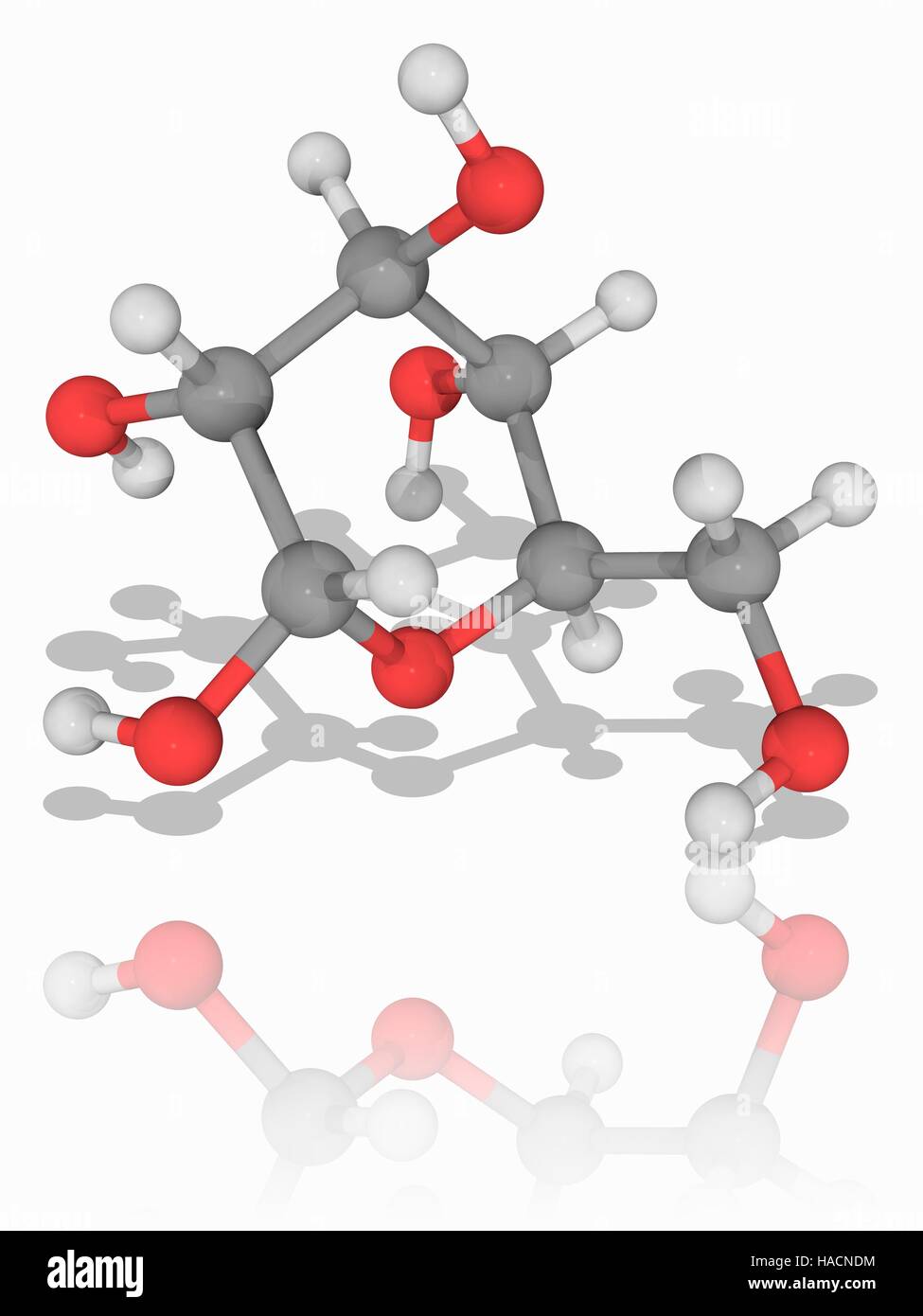
l-Glucose is an organic compound with formula C6H12O6 or O=CH[CH(OH)]5H, specifically one of the aldohexose monosaccharides. As the l-isomer of glucose, it is the enantiomer of the more common d-glucose.
l-Glucose does not occur naturally in living organisms, but can be synthesized in the laboratory. l-Glucose is indistinguishable in taste from d-glucose, but cannot be used by living organisms as a source of energy because it cannot be phosphorylated by hexokinase, the first enzyme in the glycolysis pathway. One of the known exceptions is in Burkholderia caryophylli, a plant pathogenic bacterium, which contains the enzyme d-threo-aldose 1-dehydrogenase which is capable of oxidizing l-glucose.
Like the d-isomer, l-glucose usually occurs as one of four cyclic structural isomers—α- and β-l-glucopyranose (the most common, with a six-atom ring), and α- and β-l-glucofuranose (with a five-atom ring). In water solution, these isomers interconvert in matters of hours, with the open-chain form as an intermediate stage.
Uses
l-Glucose was once proposed as a low-calorie sweetener and it is suitable for patients with diabetes mellitus, but it was never marketed due to excessive manufacturing costs.
The acetate derivative of l-glucose, l-glucose pentaacetate, was found to stimulate insulin release, and might therefore be of therapeutic value for type 2 diabetes.
l-Glucose was also found to be a laxative, and has been proposed as a colon-cleansing agent which would not produce the disruption of fluid and electrolyte levels associated with the significant liquid quantities of bad-tasting osmotic laxatives conventionally used in preparation for colonoscopy.
References
External links
- Media related to L-Glucose at Wikimedia Commons
![Glucose • einfach erklärt, D und LGlucose · [mit Video]](https://d1g9li960vagp7.cloudfront.net/wp-content/uploads/2021/04/WP_Bilder_Glucose_4-1024x576.png)